Role of the different components of an Immunoglobulin molecule
Rodney R Porter demonstrated in 1959 that the enzyme papain cleaves the Immunoglobulin molecule into three fragments each approximately 45k daltons. Each of the fragments have unique functions.
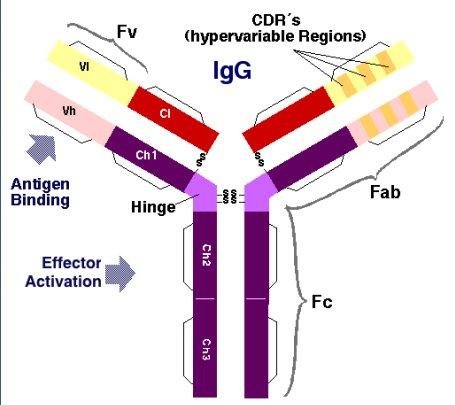
The two arms of the Y, for example, contain the site that bind antigen and, therefore, recognize specific foreign objects. This region of the antibody is called the Fab (fragment, antigen binding) region. It is composed of one constant and one variable domain from each heavy and light chain of the antibody. The paratope is shaped at the amino terminal end of the antibody monomer by the variable domains from the heavy and light chains. The variable domain is also referred to as the FV region and is the most important region for binding to antigens. More specifically variable loops, three each on the light (VL) and heavy (VH) chains are responsible for binding to the antigen. These loops are referred to as the complementarity determining regions (CDRs). In the framework of the immune network theory, CDRs are also called idiotypes. According to immune network theory, the adaptive immune system is regulated by interactions between idiotypes.
The base of the Y plays a role in modulating immune cell activity. This region is called the Fc (Fragment, crystallizable) region, and is composed of two heavy chains that contribute two or three constant domains depending on the class of the antibody.By binding to specific proteins the Fc region ensures that each antibody generates an appropriate immune response for a given antigen.The Fc region also binds to various cell receptors, such as Fc receptors, and other immune molecules, such as complement proteins. By doing this, it mediates different physiological effects including opsonization, cell lysis, and degranulation of mast cells, basophils and eosinophils.
















