Western Blotting
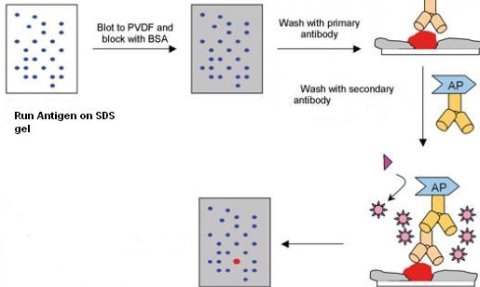
Step 1: Run the antigen on an SDS-PAGE gel and transfer contents to a PVDF or nylon membrane .Run the marker proteins on the last lane. Wet the membrane with 50% methanol.
Step 2: Block all the unbound sites with BSA or 5% milk diluted in PBS, tween 20 to avoid false positives.
Step 3: Dilute primary antibody in blocking buffer and incubate with gentle rocking on a rocker platform at 37 C for 1 hour. Wash blot 3 times for 5 minutes in wash Buffer (PBS, tween20)
Step 4: Prepare secondary antibody (Goat IgG linked to Alkaline phosphatase, 1: 5000 dilution) in blocking buffer. Incubate the blot for 1 hour at 37 C on a rocker platform. Wash blot 3 times for 5 minutes in wash Buffer (PBS, tween20).
Step 5: Mix together substrate (BCIP/NBT) 100 µl each in 10 ml substrate buffer (100 mM Tris, 100 mM NaCl, 5 mM MgCl2 pH 9.5) and soak membrane for 20 minutes. Rinse blot with deionized water for 5 minutes then air dry.
















